There is a recognized need for fabrication of highly monodisperse particles for applications such as controlled drug release and targeted drug delivery. However, the encapsulation of hydrophilic materials (such as APIs, nutraceuticals, or other compounds of interest) can be very challenging. Poly(lactic-co-glycolic acid) or PLGA is a polymer that has broad utility as vehicles for drug delivery and form the basis of several therapies approved by the US Food and Drug Administration, owing to its biodegradability and biocompatibility.
It is trivial to control the loading of the target hydrophilic material as well as the release profile of the material once encapsulated. As compared to traditional batch methods, droplet microfluidics has demonstrated that it is a very effective tool for production of PLGA particles with high monodispersity and size homogeneity.
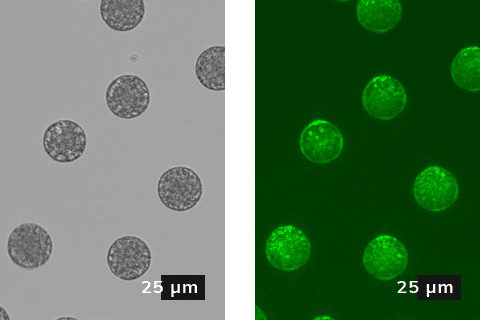
The PLGA microparticle fabrication described in this application note is ideal for the rapid iteration of formulations and optimization of the material performance characteristics. It includes details of our experimental setup, a short summary of factors influencing particle size and production rate, and the case study of fluorescent microscopy we used to analyse droplets.
Emulsion droplet size (and consequently polymer particle size) can impact how flavors and aromas are released from emulsions such as hot chocolate and mayonnaise. Size also impacts the speed at which drugs or agrochemicals are delivered and released, and the length of time needed between treatments.
Microfluidics provides a tool to manipulate liquids, gases, droplets, cell and particles within micro-channel geometries. The generation of droplets involves controlling the jetting to dripping transition when liquid droplets are pushed into a carrier fluid via a specific chip geometry. The droplets are stabilized using surfactants to avoid coagulation and separation.
Among its various advantages, microfluidic technology has the ability to create three-dimensional flow patterns that achieve precise control over immiscible and miscible fluid mixing.
Drug molecules or the active pharmaceutical ingredient (API) encapsulation in PLGA particles is commonly used to create specific particles for personalized medicines, API solubilization and controlled release. More and more scientists adopt microfluidic methods which generate highly monodisperse particles in a single continuous step, increasing the yield of the process and achieving the specific particle properties without a significant loss of the sample.
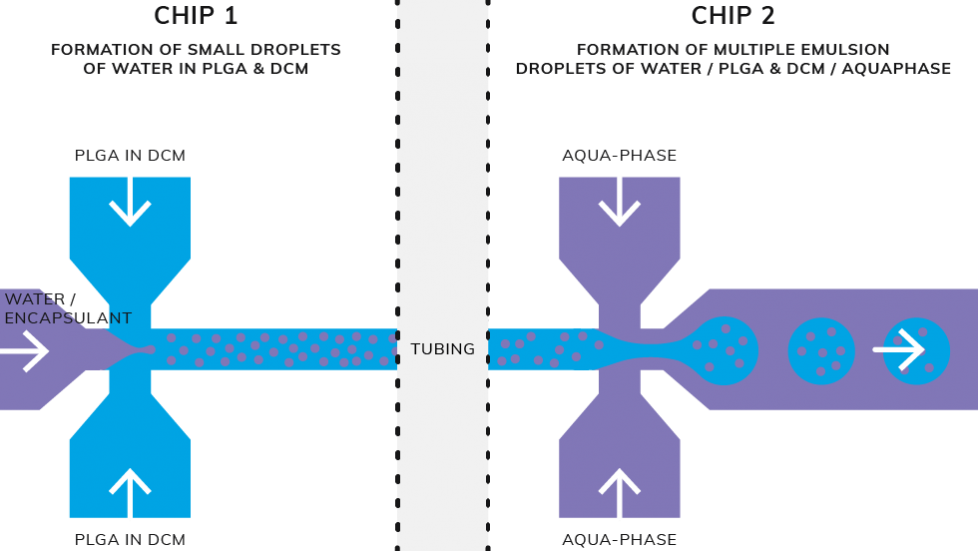
A microfluidic droplet production method for polymer multiple emulsions is a two step continuous process. Stage 1) the emulsification of an aqueous solution in an organic continuous phase; Stage 2) the emulsification of this primary emulsion in a second aqueous phase (containing an emulsion stabilizer). The result is a water-in-oil-in-water emulsion and once formed, this emulsion is dried to produce monodisperse PLGA microparticles containing multiple aqueous droplets. The size and production throughput can be readily tuned by adjusting the flow rates at any continuous process stages.
Dolomite’s Drug (API) Encapsulation Systems are the perfect solution for this pharmaceutical application as they utilize microfluidic methods to directly generate monodisperse particles, beads and emulsions, eliminating the need for further processing. They can be used to generate particles in the micro size range, resulting in formulations such as water in oil (w/o) or oil in water (o/w) emulsions.
Methodology for generation of monodisperse multiple emulsions in sizes ranging from 20 to 50 μm using droplet microfluidics.