Polyacrylamide (PAM) is a type of hydrogel consisting of a loosely cross-linked acrylamide structure that can hold large amount of water. Due to their high water content, polyacrylamide is also highly amenable for use as biomaterial that can be in contact with tissue or biological fluids as implants, soft contact lenses, and drug delivery particle systems. Polyacrylamide beads can be readily surface functionalized and have been recently used in a wide range of microencapsulation applications including RNA capture, drug encapsulation, controlled release, and enzyme immobilization.
Microfluidic technology for polyacrylamide synthesis has seen a significant growth in various fields. High reproducibility, real-time control and reduction of waste are the main factors that push users to switch from conventional batch methods to microfluidics.
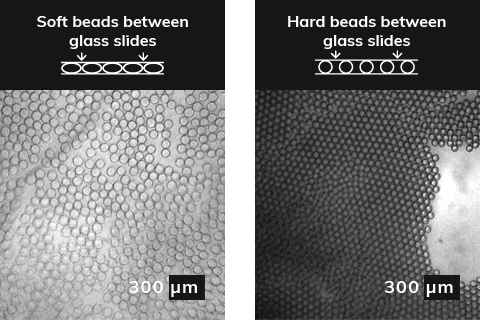
The growing need for specific polyacrylamide beads becomes more dominant with a wide range of applications available across various industries. For example, biologists and researchers use polyacrylamide soft deformable (soft) beads that are close packed to insert a controllable number of particles in every drop. Microfluidic devices enable to load beads with discrete objects, such as particles and cells, which is often needed when performing chemical and biological assays. Not only it improves efficiency of loading techniques and encapsulation yields, it also provides a full control over particle size and wastage management.
In a single word, yes! The porous polyacrylamide structure allows growth materials transferred and exchanged via the nanopores in the bead structure. Bead size can impact the speed of cell growth and the degradation speed at which drugs or agrochemicals are delivered and released, impacting the length of time needed between treatments.
Microfluidics provides a tool to manipulate liquids, gases, droplets, cell and particles within micro-channel geometries. The generation of droplets involves controlling the jetting to dripping transition when liquid droplets are pushed into a carrier fluid via a specific chip geometry. The droplets are stabilized using surfactants to avoid coagulation and separation.
Among its various advantages, microfluidic technology has the ability to create three-dimensional flow patterns that achieve precise control over immiscible and miscible fluid mixing.
Droplet-based microfluidic systems have shown exceptional advantages for the synthesis of monomer- or polymer-containing droplets inside which the hydrogel structures can be formed using various chemical methods. By precisely controlling the formation of the bead, we can produce polyacrylamide particles with well-defined sizes, shapes and morphologies.
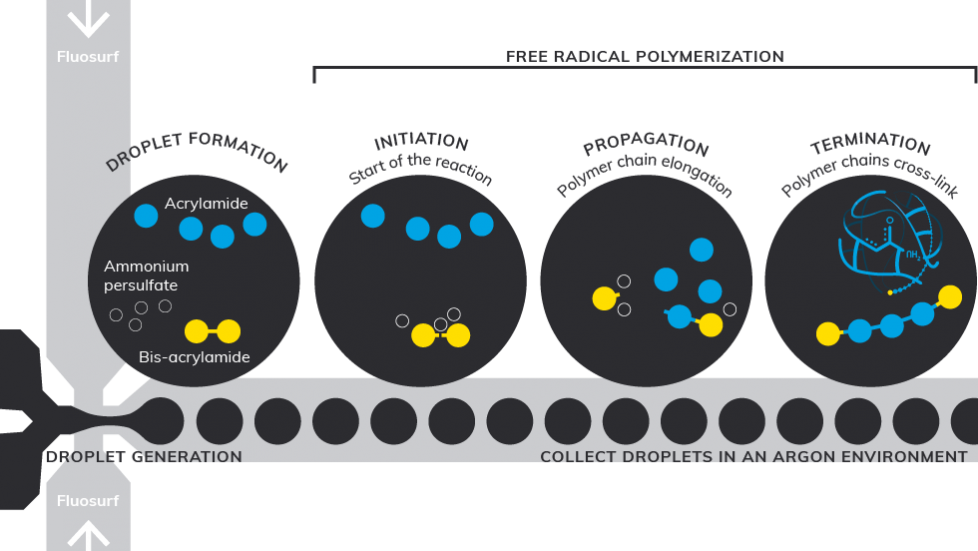
The schematics below showcases the flow focusing microfluidic method example from our application note. Polyacrylamide hydrogel beads are produced by free radical copolymerization of acrylamide and bis-acrylamide initiated by ammonium persulfate. The persulfate free radicals convert acrylamide monomers to free radicals which react with inactivated monomers to begin the polymerization chain reaction. The bead characteristics is resulted by the randomly crosslinked polymer chains, which are influenced by polymerization conditions and monomer concentrations.

Telos® High-Throughput Production Systems provide a controlled solution for scaling-up droplet, particles or emulsion manufacturing. Each Dolomite Microfluidic System has a flexible configuration which allows up to 10 Telos®Clamp Modules to be assembled in parallel, enabling identical conditions for scale-up of production rate for up to 500,000 monodisperse particles per second.
Methodology for generation of monodisperse polyacrylamide beads in sizes ranging from 20 to 220 µm using microfluidic flow focusing method.

Read the latest featured story about how our experts are using microfluidics to produce small (80-120 µm) alginate beads suitable for cell encapsulation.
Read more about the emulsion stabilizer used in our experiments with polyacrylamide. FluoSurf is an emulsion stabiliser for aqueous droplets in fluorinated oil.
Methodology for monodisperse agarose particle production using microfluidic droplet methods, with particle sizes varied between 20 µm to 130 µm.